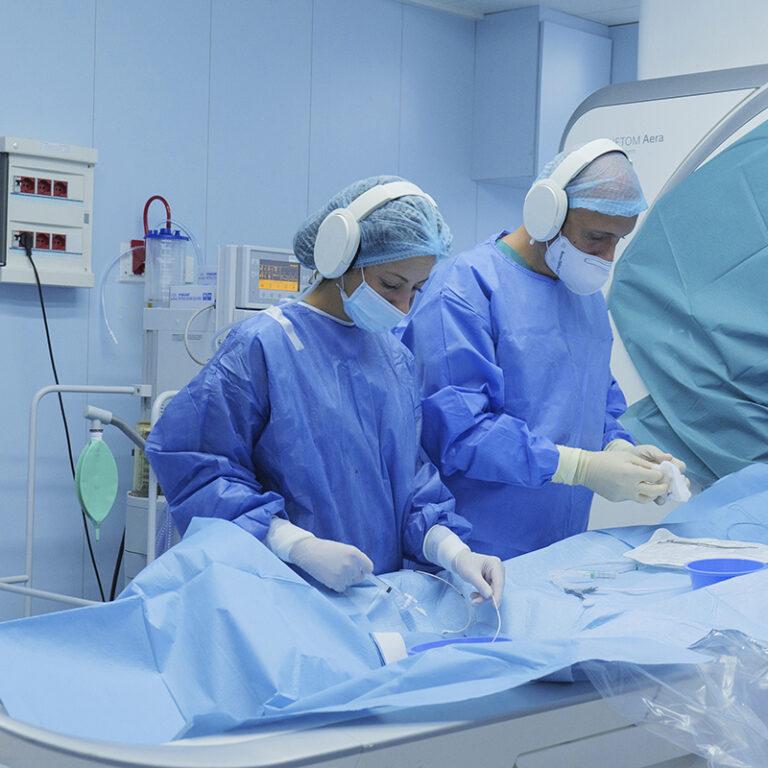
CAR-T
THERAPY
THE
CONTEXT
Unfortunately, many children suffering from serious oncological conditions do not respond to traditional treatments such as radiotherapy, chemotherapy, or bone marrow transplantation.
However, thanks to the progress of scientific research, there is hope for these children through gene therapy or immunotherapy, which involves modifying the patient’s immune system cells to recognize and attack tumors.
This personalized medicine approach, known as CAR-T (Chimeric Antigen Receptor T-cell), has shown extraordinary results in treating certain types of cancer, particularly leukemia and lymphoma.
CAR-T is authorized in the European Union for specific hematological malignancies and Bambino Gesù Children’s Hospital, the largest pediatric institution in Europe, is the sole Italian hospital that produces and administers CAR-T therapies.
In Italy, CAR-T therapy is classified as experimental, and Ospedale Bambino Gesù administers it with AIFA authorization. This authorization allows for the administration of CAR-T therapy within clinical trials or under the category of “exceptional non-repetitive use” when all conventional treatment options have been exhausted.
THE
CONCEPT
Recent studies conducted at Bambino Gesù Children’s Hospital, published in the New England Journal of Medicine (April 2023), have demonstrated the effectiveness of CAR-T in treating Neuroblastoma, a solid tumor affecting both children and adults.
This breakthrough could potentially replace chemotherapy in treating various solid tumors, revolutionizing oncological treatments.
These results are the outcome of top-level research conducted by our hospital, made possible by our investment in a dedicated Pharmaceutical Workshop. We have 1,400sqm of research laboratories focused on generating cell and gene therapy products.
The production of CAR-T therapy costs around 90,000 euros per patient. Currently, there is no reimbursement available for non-repetitive use of CAR-T, and the hospital bears the full cost. The demand for access to these experimental treatments is growing rapidly across Europe, and families from all over the continent are seeking treatment at Bambino Gesù hospital.
WHAT WILL
WE DO
This year, we aim to treat approximately 80 children with CAR-T therapy. To make treatment accessible and ensure every child with a treatable serious condition can receive CAR-T, we have established the Bambino Gesù Humanitarian Fund for CAR-T. This fund supports the production and administration costs of this expensive therapy but, unfortunately, the fund for 2023 has been depleted due to the growing demand.
Every child deserves immediate access to treatment.
You can support this project by donating and ensuring that all children in need will have access to this life-changing therapy.